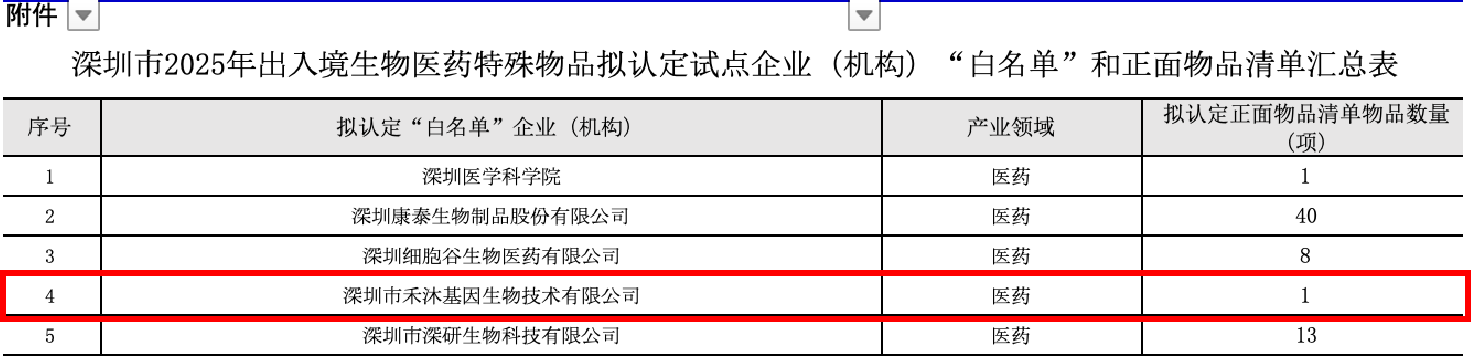
Obtaining this “White List” qualification signifies official recognition of Hemogen Therapeutic’s compliance capabilities in cross-border cellular and genetic material logistics. This approval provides a regulatory foundation for establishing a fully compliant international workflow of “patient samples in, cell products out,” enabling an integrated cross-border treatment pathway.
The qualification will significantly improve the efficiency of overseas patient samples entering China for process verification, cell manufacturing, and quality testing. It also supports a dual-channel development model—clinical validation abroad and manufacturing/technical verification in China—thereby accelerating multicountry clinical data generation and regulatory submissions. At the same time, the enhanced cross-border transport capability will directly facilitate the upcoming clinical studies in Hong Kong by strengthening the timeliness and stability of sample movement under the Shenzhen–Hong Kong collaborative framework. It will also provide smoother sample-flow pathways for collaborations underway in Saudi Arabia, Jordan, Bahrain, and other Middle Eastern countries, helping advance the implementation of local gene-therapy programs.
In alignment with Hemogen Therapeutic’s ongoing international strategy—anchored by the HGI-001 (Hemocel) program, which has already entered clinical development in Thailand, and the company’s “License-out + Technology Transfer” global expansion model—the newly obtained qualification will further enhance worldwide access to China-developed gene-therapy innovations. It will also support the establishment of standardized and localized gene-therapy service networks across Belt and Road regions and other areas with a high prevalence of inherited diseases.